Introduction: Acute myeloid leukemia (AML) is associated with poor prognosis, especially in elderly patients unfit for intensive chemotherapy. AML chemotherapy agents, including venetoclax, a BH3-mimetic, are designed to induce cell death through apoptosis. Unfortunately, AML cells eventually develop a resistance to BCL-2-dependent apoptosis which causes clinical disease recurrence. Alternative cell death pathways like ferroptosis and necroptosis are lytic forms of cell death mediated by plasma membrane lipid oxidation or MLKL phosphorylation (p-MLKL), respectively, both of which disrupt plasma membrane integrity. Because apoptosis and ferroptosis involve different molecular pathways, ferroptosis-inducing therapies provide an opportunity to overcome therapy resistance in AML patients. Here, we show how necroptosis inhibitor targeting basal necroptosis signal selectively induces ferroptosis in venetoclax-resistant AML cells causing cell death.
Methods: We established venetoclax-resistant myeloid cell lines by exposing cell lines to increasing doses of venetoclax. We evaluated resistance to venetoclax-induced apoptosis by measuring IC50 after Annexin-V/PI staining. We screened venetoclax-resistant myeloid cell lines and primary AML cells (n=77) for basal activation of necroptosis by pMLKL or phosphorylated RIP3 (pRIP3) western blotting. Xenotransplantation was performed to evaluate the bone marrow engraftment potentials of basal activated necroptosis primary AMLs. Necrosome inhibitor (Zharp-99) was tested to estimate IC50 for cell death induction in myeloid cell lines after Annexin-V/PI staining. Ferroptosis inhibitors (ferrostatin-1 and lipoxstatin-1) and apoptosis inhibitor (zVAD-FMK) were used to determine which death pathway was induced by necroptosis inhibitors in AML.
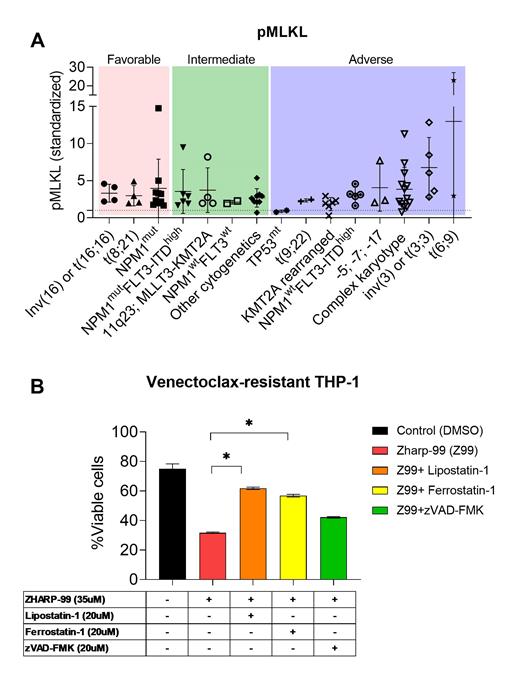
Results: We established venetoclax-resistant myeloid cell lines (THP1-VR and HL60-VR), which showed a significant shift in IC50 for venetoclax-induced apoptosis compared to parent cell lines. Venetoclax-resistant cell lines acquired an upregulation of known anti-apoptotic proteins; BCL-2, BCL-XL, and MCL-1. Venetoclax-resistant cell lines upregulated pRIP3 signal, a known necroptosis driver, by western blot. Necrosome screening of primary AML samples revealed 73 AMLs (94%) showed basal necroptosis signal confirmed by pMLKL with heterogeneous intensities (Figure. A). Xenotransplantation confirmed engraftment of basal necroptosis-activated primary AMLs is achievable and with retention of necroptosis signal at engraftment. The basal necroptosis signal in venetoclax-resistant cell lines and primary AML samples drove us to evaluate necroptosis inhibitor Zharp-99 (RIP3K inhibitor) IC50 for venetoclax-resistant cell lines. Compared to parent cell lines, venetoclax-resistant cell lines became susceptible to cell death by necrosome inhibition. Surprisingly, the cell death induced by Zharp-99 was not from apoptosis but through ferroptosis, confirmed by the venetoclax-resistant myeloid cells being rescued from cell death by ferroptosis inhibitor (ferrostatin-1 and lipoxstatin-1) (Figure B).
Conclusion: Basal activation of necrosome is observed in venetoclax-resistant myeloid cell lines and primary AML patient samples. We found necroptosis inhibitor Zharp-99 promoted cell death in necrosome-activated venetoclax-resistant cell lines. Our data suggest necroptosis inhibition targeting RIP3K activity has a novel function of inducing ferroptosis in myeloid malignancies resistant to apoptosis. RIP3K inhibitor, as a novel function of ferroptosis inducer in apoptosis-resistant myeloid cell lines, opens a new therapy opportunity to treat apoptosis resistance in AML patients. We plan to investigate in vivo effects of necrosome inhibitors using our xenotransplant model.
Disclosures
Ito:Horizon Therapeutics: Other: Clinical trial drug supply ; BlueSphere Bio: Patents & Royalties: Patent , Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal